Abstract
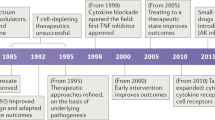
The introduction of biologic agents to clinical practice has had a major bearing on the treatment of patients with chronic inflammatory diseases such as rheumatoid arthritis. These drugs have the potential to improve the outcome of disease and the quality of life for patients. However, clinical criteria alone are inadequate for determining which therapy is most appropriate for an individual patient. Furthermore, why a particular drug is effective in a particular patient, or indeed in any patient, but is ineffective for other individuals, is often unknown. In this Review, we provide an overview of biologic therapies currently available for patients with rheumatoid arthritis, and discuss why certain immunological regulators represent potential targets for intervention. Current agents can be clustered into three major types: cytokine blockers, lymphocyte-targeting agents, and small-molecule inhibitors of signal transduction pathways. We differentiate among the modes of action of each of these types of therapy and consider the challenges associated with their use in clinical practice.
Key Points
-
Current interventions in rheumatoid arthritis (RA) can be classified into three major types: cytokine blockers, lymphocyte-targeting agents, and small-molecule inhibitors of signal transduction pathways
-
In the absence of reliable biomarkers, mode of action could be a useful tool in guiding choice of biologic therapy
-
Adverse effects of biologic agents are either class specific or compound specific; class-specific effects reflect the biological role of the target
-
Early-phase clinical trials in RA in the past few years have mostly focused on cytokine blockers and small-molecule inhibitors of signal transduction pathways
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Singh, J. A. et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 64, 625–639 (2012).
Smolen, J. S. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann. Rheum. Dis. 69, 964–975 (2010).
Singh, J. A. et al. Biologics for rheumatoid arthritis: an overview of Cochrane reviews. Sao Paulo Med. J. 128, 309–310 (2010).
Smolen, J. S. et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet 374, 210–221 (2009).
Cohen, S. B. et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 54, 2793–2806 (2006).
Genovese, M. C. et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N. Engl. J. Med. 353, 1114–1123 (2005).
Emery, P. et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann. Rheum. Dis. 67, 1516–1523 (2008).
Molenaar, E. T. et al. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum. 50, 36–42 (2004).
Furst, D. E. et al. Adalimumab, a fully human anti tumor necrosis factor-α monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis). J. Rheumatol. 30, 2563–71 (2003).
Klareskog, L., Catrina, A. I., Paget, S. Rheumatoid arthritis. Lancet 373, 659–672 (2009).
Isaacs, J. D. et al. Effect of baseline rheumatoid factor and anti-citrullinated peptide antibody serotype on rituximab clinical response: a meta-analysis. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2011-201117
Bugatti, S. A. et al. Synovial tissue heterogeneity and peripheral blood biomarkers. Curr. Rheumatol. Rep. 13, 440–448 (2011).
de Hair, M. J. et al. Synovial tissue analysis for the discovery of diagnostic and prognostic biomarkers in patients with early arthritis. J. Rheumatol. 38, 2068–2072 (2011).
van de Sande, M. G. et al. Evaluating antirheumatic treatments using synovial biopsy: a recommendation for standardization to be used in clinical trials. Ann. Rheum. Dis. 70, 423–427 (2011).
McInnes, I. B. & O'Dell, J. R. State-of-the-art: rheumatoid arthritis. Ann. Rheum. Dis. 69, 1898–1906 (2010).
Stranger, B. E., Stahl, E. A. & Raj, T. Progress and promise of genome-wide association studies for human complex trait genetics. Genetics 187, 367–383 (2011).
Feldmann, M., Brennan, F. M., Maini, R. N. Role of cytokines in rheumatoid arthritis. Ann. Rev. Immunol. 14, 397–440 (1996).
Stahl, E. A. et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat. Gen. 42, 508–514 (2010).
Woodrick, R. S. & Ruderman, E. M. Safety of biologic therapy in rheumatoid arthritis. Nat. Rev. Rheumatol. 7, 639–652 (2011).
Choy, E. H. & Panayi, G. S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 344, 907–916 (2001).
McInnes, I. B. & Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 7, 429–442 (2007).
Dayer, J. M. & Choy, E. Therapeutic targets in rheumatoid arthritis: the interleukin-6 receptor. Rheumatology 49, 15–24 (2010).
Raison, C. L., Capuron, L. & Miller, A. H. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 27, 24–31 (2006).
Kirkham, B. Interleukin-1, immune activation pathways, and different mechanisms in osteoarthritis and rheumatoid arthritis. Ann. Rheum. Dis. 50, 395–400 (1991).
Carroll, G., Bell, M., Wang, H., Chapman, H. & Mills, J. Antagonism of the IL-6 cytokine subfamily—a potential strategy for more effective therapy in rheumatoid arthritis. Inflamm. Res. 47, 1–7 (1998).
Busso, N. & So, A. Mechanisms of inflammation in gout. Arthritis Res. Ther. 12, 206 (2010).
Aaltonen, K. J. et al. Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. PLoS ONE 7, e30275 (2012).
Singh, J. A., Beg, S. & Lopez-Olivo, M. A. Tocilizumab for rheumatoid arthritis: a Cochrane systematic review. J. Rheumatol. 38, 10–20 (2011).
Brennan, F. M., Maini, R. N. & Feldmann, M. TNF α--a pivotal role in rheumatoid arthritis? Br. J. Rheumatol. 31, 293–298 (1992).
Yokota, S. et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 371, 998–1006 (2008).
Silke, J. The regulation of TNF signalling: what a tangled web we weave. Curr. Opin. Immunol. 23, 620–626 (2011).
Kamimura, D., Ishihara, K. & Hirano, T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev. Physiol. Biochem. Pharmacol. 149, 1–38 (2003).
Nowell, M. A. et al. Therapeutic targeting of IL-6 trans signaling counteracts STAT3 control of experimental inflammatory arthritis. J. Immunol. 182, 613–622 (2009).
de Hooge, A. S. et al. Local activation of STAT-1 and STAT-3 in the inflamed synovium during zymosan-induced arthritis: exacerbation of joint inflammation in STAT-1 gene-knockout mice. Arthritis Rheum. 50, 2014–2023 (2004).
Hayden, M. S. & Ghosh, S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 26, 203–234 (2012).
Cheon, H., Yang, J. & Stark, G. R. The functions of signal transducers and activators of transcriptions 1 and 3 as cytokine-inducible proteins. J. Interferon Cytokine Res. 31, 33–40 (2011).
Jones, S. A., Scheller, J. & Rose-John, S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J. Clin. Invest. 121, 3375–3383 (2011).
Hsu, B., Sheng, S., Smolen, J. & Weinblatt, M. Results from a 2-part, proof-of-concept, dose-ranging, randomized, double-blind, placebo-controlled, phase 2 study of sirukumab, a human anti-interleukin-6 monoclonal antibody, in active rheumatoid arthritis patients despite methotrexate therapy. Arthritis Rheum. 63 (Suppl.), S1034 (2011).
Hickling, M. et al. Safety and pharmacokinetics of CDP6038, an anti-IL-6 monoclonal antibody, administered by subcutaneous injection and intravenous infusion to healthy male volunteers: a phase 1 study. Ann. Rheum. Dis. 70 (Suppl. 3), 471 (2011).
Mease, P. et al. A phase II, double-blind, randomised, placebo-controlled study of BMS945429 (ALD518) in patients with rheumatoid arthritis with an inadequate response to methotrexate. Ann. Rheum. Dis. 71, 1183–1189 (2012).
Radin, A. et al. Safety and effects on markers of inflammation of subcutaneously administered regn88/sar153191 (regn88), an interleukin-6 receptor inhibitor, in patients with rheumatoid arthritis: findings from phase 1 studies. Ann. Rheum. Dis. 69 (Suppl. 3), 99 (2010).
Lissilaa, R. et al. Although IL-6 trans-signaling is sufficient to drive local immune responses, classical IL-6 signaling is obligate for the induction of T cell-mediated autoimmunity. J. Immunol. 185, 5512–5521 (2010).
Richards, P. J. et al. Functional characterization of a soluble gp130 isoform and its therapeutic capacity in an experimental model of inflammatory arthritis. Arthritis Rheum. 54, 1662–1672 (2006).
Hueber, W. et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci. Transl. Med. 2, 52–72 (2010).
Papp, K. A. et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N. Engl. J. Med. 366, 1181–1189 (2012).
Gandhi, M. et al. Anti-p40 antibodies ustekinumab and briakinumab: blockade of interleukin-12 and interleukin-23 in the treatment of psoriasis. Semin. Cutan. Med. Surg. 29, 48–52 (2010).
Hunter, C. A. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 5, 521–531 (2005).
Murphy, K. M. & Stockinger, B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 11, 674–680 (2010).
Mukasa, A. et al. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity 32, 616–627 (2010).
Wei, G. et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167 (2009).
Lee, K. Y. et al. Late developmental plasticity in the T helper 17 lineage. Immunity 30, 92–107 (2009).
Zhu, J. & Paul, W. E. CD4+ T cell plasticity—TH2 cells join the crowd. Immunity 32, 11–13 (2010).
Zhou, L. et al. Plasticity of CD4+ T cell lineage differentiation. Immunity 30, 646–655 (2009).
Cornelissen, F. et al. Interleukin-23 is critical for full-blown expression of a non-autoimmune destructive arthritis and regulates interleukin-17A and RORγt in γδ T cells. Arthritis Res. Ther. 11, R194 (2009).
Cragg, M. S., Walshe, C. A., Ivanov, A. O. & Glennie, M. J. The biology of CD20 and its potential as a target for mAb therapy. Curr. Dir. Autoimmun. 8, 140–174 (2005).
Edwards, J. C., Leandro, M. J. & Cambridge, G. B lymphocyte depletion in rheumatoid arthritis: targeting of CD20. Curr. Dir. Autoimmun. 8, 175–192 (2005).
Vos, K. et al. Early effects of rituximab on the synovial cell infiltrate in patients with rheumatoid arthritis. Arthritis Rheum. 56, 772–778 (2007).
Teng, Y. K. et al. Immunohistochemical analysis as a means to predict responsiveness to rituximab treatment. Arthritis Rheum. 56, 3909–3918 (2007).
Vital, E. M. et al. Management of nonresponse to rituximab in rheumatoid arthritis: predictors and outcome of re-treatment. Arthritis Rheum. 62, 1273–1279 (2010).
Dorner, T., Kinnman, N. & Tak, P. P. Targeting B cells in immune-mediated inflammatory disease: a comprehensive review of mechanisms of action and identification of biomarkers. Pharmacol. Ther. 125, 464–475 (2010).
Kausar, F. et al. Ocrelizumab: a step forward in the evolution of B-cell therapy. Exp. Opin. Biol. Ther. 9, 889–895 (2009).
Goldenberg, D. M., Morschhauser, F. & Wegener, W. A. Veltuzumab (humanized anti-CD20 monoclonal antibody): characterization, current clinical results, and future prospects. Leuk. Lymphoma. 51, 747–755 (2010).
Taylor, P. C. et al. Ofatumumab, a fully human anti-CD20 monoclonal antibody, in biological-naive, rheumatoid arthritis patients with an inadequate response to methotrexate: a randomised, double-blind, placebo-controlled clinical trial. Ann. Rheum. Dis. 70, 2119–2125 (2011).
Dorner, T. & Goldenberg, D. M. Targeting CD22 as a strategy for treating systemic autoimmune diseases. Ther. Clin. Risk Manage. 3, 953–959 (2007).
Tak, P. P. et al. Atacicept in patients with rheumatoid arthritis: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating, single- and repeated-dose study. Arthritis Rheum. 58, 61–72 (2008).
Cohen, S. B. Updates from B cell trials: efficacy. J. Rheumatol. 77 (Suppl.), 12–17 (2006).
van Vollenhoven, R. F., Kinnman, N., Vincent, E., Wax, S. & Bathon, J. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase II, randomized, placebo-controlled trial. Arthritis Rheum. 63, 1782–1792 (2011).
Genovese, M. C., Kinnman, N., de La Bourdonnaye, G., Pena Rossi, C. & Tak, P. P. Atacicept in patients with rheumatoid arthritis and an inadequate response to tumor necrosis factor antagonist therapy: results of a phase II, randomized, placebo-controlled, dose-finding trial. Arthritis Rheum. 63, 1793–1803 (2011).
Shevach, E. M. Immunology: regulating suppression. Science 322, 202–203 (2008).
Lenschow, D. J. & Bluestone, J. A. T cell co-stimulation and in vivo tolerance. Curr. Opin. Immunol. 5, 747–752 (1993).
Buch, M. H. et al. Mode of action of abatacept in rheumatoid arthritis patients having failed tumour necrosis factor blockade: a histological, gene expression and dynamic magnetic resonance imaging pilot study. Ann. Rheum. Dis. 68, 1220–1227 (2009).
Ko, H. J. et al. CTLA4-Ig modifies dendritic cells from mice with collagen-induced arthritis to increase the CD4+CD25+Foxp3+ regulatory T cell population. J. Autoimmun. 34, 111–120 (2010).
Choy, E. H. Selective modulation of T-cell co-stimulation: a novel mode of action for the treatment of rheumatoid arthritis. Clin. Exp. Rheumatol. 27, 510–518 (2009).
Remans, P. H. et al. CTLA-4IG suppresses reactive oxygen species by preventing synovial adherent cell-induced inactivation of Rap1, a Ras family GTPASE mediator of oxidative stress in rheumatoid arthritis T cells. Arthritis Rheum. 54, 3135–3143 (2006).
Alvarez-Quiroga, C. et al. CTLA-4-Ig therapy diminishes the frequency but enhances the function of TREG cells in patients with rheumatoid arthritis. J. Clin. Immunol. 31, 588–595 (2011).
Platt, A. M. et al. Abatacept limits breach of self-tolerance in a murine model of arthritis via effects on the generation of T follicular helper cells. J. Immunol. 185, 1558–1567 (2010).
Choy, E. H. et al. Repeat-cycle study of high-dose intravenous 4162W94 anti-CD4 humanized monoclonal antibody in rheumatoid arthritis. A randomized placebo-controlled trial. Rheumatology (Oxford) 41, 1142–1148 (2002).
Yocum, D. E. et al. Clinical and immunologic effects of a PRIMATIZED anti-CD4 monoclonal antibody in active rheumatoid arthritis: results of a phase I, single dose, dose escalating trial. J. Rheumatol. 25, 1257–1262 (1998).
Isaacs, J. D. Therapeutic T-cell manipulation in rheumatoid arthritis: past, present and future. Rheumatology 47, 1461–1468 (2008).
O'Shea, J. J., Park, H., Pesu, M., Borie, D. & Changelian, P. New strategies for immunosuppression: interfering with cytokines by targeting the Jak/Stat pathway. Curr. Opin. Rheumatol. 17, 305–311 (2005).
Ghoreschi, K., Laurence, A. & O'Shea, J. J. Janus kinases in immune cell signaling. Immunol. Rev. 228, 273–287 (2009).
Jiang, H. et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343–1355 (2009).
Fleischmann, R. Novel small-molecular therapeutics for rheumatoid arthritis. Curr. Opin. Rheumatol. 24, 335–341 (2012).
O'Shea, J. J. & Murray, P. J. Cytokine signaling modules in inflammatory responses. Immunity. 28, 477–487 (2008).
Burmester, G. R. et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet http://dx.doi.org/10.1016/S0140-6736(12)61424-X.
Yoshida, H. et al. Low dose CP-690, 550 (tofacitinib), a pan-JAK inhibitor, accelerates the onset of experimental autoimmune encephalomyelitis by potentiating TH17 differentiation. Biochem. Biophys. Res. Commun. 418, 234–240 (2012).
Sesin, C. A. & Bingham, C. O. 3rd. Remission in rheumatoid arthritis: wishful thinking or clinical reality? Semin. Arthritis Rheum. 35, 185–196 (2005).
Katchamart, W. Predictors for remission in rheumatoid arthritis patients: a systematic review. Arthritis Care Res. 62, 1128–1143 (2010).
Genovese, M. C. et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 50, 1412–1419 (2004).
Weinblatt, M. et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann. Rheum. Dis. 66, 228–234 (2007).
Dépis, F. et al. Long term amelioration of established collagen-induced arthritis achieved with short term therapy combining anti-CD3 and anti-TNF treatments. Arthritis Rheum. 64, 3189–3198 (2012).
Acknowledgements
Professors Ernest Choy and Simon Jones thank Arthritis Research UK for funding support provided through the CREATE Centre (20016) and research grants (19796, 19381, 18286).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to researching data for the article and writing the article. E. H. Choy handled review and editing of the article before submission.
Corresponding author
Ethics declarations
Competing interests
E. H. Choy declares that he has received research grants from and has served as a member of advisory boards and speakers bureaus for Abbott, Allergan, AstraZeneca, Boehringer Ingelheim, Chelsea Therapeutics, Chugai Pharma, Eli Lilly, GlaxoSmithKline, Jazz Pharmaceuticals, Merrimack Pharmaceutical, Merck Sharp & Dohme, Pfizer, Pierre Fabre Medicament, Roche, Schering Plough, Synovate, UCB, and Wyeth. A. F. Kavanaugh declares that he has received research grants from Abbott, Amgen, Bristol-Myers Squibb, Janssen, Pfizer, Roche, and UCB. S. A. Jones declares that he has consultancy agreements with F. Hoffmann-La Roche and NovImmune SA and has acted as an advisor for Chugai Pharma Europe and Genentech. He is also recipient of an investigator-sponsored award from F. Hoffmann-La Roche.
Rights and permissions
About this article
Cite this article
Choy, E., Kavanaugh, A. & Jones, S. The problem of choice: current biologic agents and future prospects in RA. Nat Rev Rheumatol 9, 154–163 (2013). https://doi.org/10.1038/nrrheum.2013.8
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2013.8
This article is cited by
-
Rspo2 exacerbates rheumatoid arthritis by targeting aggressive phenotype of fibroblast-like synoviocytes and disrupting chondrocyte homeostasis via Wnt/β-catenin pathway
Arthritis Research & Therapy (2023)
-
Vaccines prevent reinduction of rheumatoid arthritis symptoms in collagen-induced arthritis mouse model
Drug Delivery and Translational Research (2023)
-
Perceptions towards biologic and biosimilar therapy of patients with rheumatic and gastroenterological conditions
BMC Rheumatology (2022)
-
Histone methyltransferase EZH2 in proliferation, invasion, and migration of fibroblast-like synoviocytes in rheumatoid arthritis
Journal of Bone and Mineral Metabolism (2022)
-
Targeting angiogenesis for fracture nonunion treatment in inflammatory disease
Bone Research (2021)