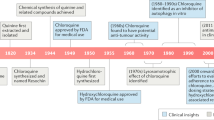
Abstract
Antimalarial agents have routinely been used for the treatment of systemic lupus erythematosus (SLE) for over 50 years. These agents continue to enjoy success as the initial pharmacotherapy for SLE even in the era of targeted therapies. Antimalarial agents have numerous biological effects that are responsible for their immunomodulatory actions in SLE. Their inhibitory effect on Toll-like receptor-mediated activation of the innate immune response is perhaps the most important discovery regarding their putative mechanism of action, but some other, previously known properties, such as antithrombotic and antilipidaemic effects, are now explained by new research. In the 1980s and 1990s, these antihyperlipidaemic and antithrombotic effects were demonstrated in retrospective clinical studies, and over the past few years prospective studies have confirmed those findings. Knowledge about the risk–benefit profile of antimalarial agents during pregnancy and lactation has evolved, as has the concept of retinal toxicity. Antimalarial agents have unique disease-modifying properties in SLE and newer iterations of this class of anti-inflammatory agents will have a profound effect upon the treatment of autoimmune disease.
Key Points
-
Antimalarial agents are the cornerstone agents in the clinical management of systemic lupus erythematosus
-
Toll-like receptor (TLR)-antagonism has emerged as an important mechanism of action of antimalarial agents
-
The antilipidaemic, photoprotective and antiproliferative effects of chloroquine, hydroxychloroquine and quinacrine are in part explained by TLR antagonism
-
Antimalarial agents also act by several additional molecular mechanisms, the understanding of which continues to evolve
-
Antimalarial agents are generally safe, effective and clinically useful in almost all patients with systemic lupus erythematosus
-
These drugs offer considerable promise for treating a variety of immune-mediated as well as nonimmune diseases, and have exciting potential
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wallace, D. J. The history of antimalarials. Lupus 5 (Suppl. 1), S2–S3 (1996).
Knox, C. et al. DrugBank 3.0: a comprehensive resource for 'omics' research on drugs. Nucleic Acids Res. 39, D1035–D1041 (2011).
Tett, S. E., McLachlan, A. J., Cutler, D. J. & Day, R. O. Pharmacokinetics and pharmacodynamics of hydroxychloroquine enantiomers in patients with rheumatoid arthritis receiving multiple doses of racemate. Chirality 6, 355–359 (1994).
Furst, D. E. Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus 5 (Suppl. 1), S11–S15 (1996).
Scherbel, A. L., Schuchter, S. L. & Harrison, J. W. Comparison of effects of two antimalarial agents, hydroxychloroquine sulfate and chloroquine phosphate, in patients with rheumatoid arthitis. Cleve. Clin. Q. 24, 98–104 (1957).
Block, J. A. Hydroxychloroquine and retinal safety. Lancet 351, 771 (1998).
Costedoat-Chalumeau, N. et al. Low blood concentration of hydroxychloroquine is a marker for and predictor of disease exacerbations in patients with systemic lupus erythematosus. Arthritis Rheum. 54, 3284–3290 (2006).
Costedoat-Chalumeau, N. et al. Very low blood hydroxychloroquine concentration as an objective marker of poor adherence to treatment of systemic lupus erythematosus. Ann. Rheum. Dis. 66, 821–824 (2007).
Wallace, D. J. Antimalarials—the 'real' advance in lupus. Lupus 10, 385–387 (2001).
Fox, R. I. Mechanism of action of hydroxychloroquine as an antirheumatic drug. Semin. Arthritis Rheum. 23, 82–91 (1993).
Wallace, D. J., Linker-Israeli, M., Hyun, S., Klinenberg, J. R. & Stecher, V. The effect of hydroxychloroquine therapy on serum levels of immunoregulatory molecules in patients with systemic lupus erythematosus. J. Rheumatol. 21, 375–376 (1994).
Wozniacka, A., Lesiak, A., Narbutt, J., McCauliffe, D. P. & Sysa-Jedrzejowska, A. Chloroquine treatment influences proinflammatory cytokine levels in systemic lupus erythematosus patients. Lupus 15, 268–275 (2006).
Jang, C. H., Choi, J. H., Byun, M. S. & Jue, D. M. Chloroquine inhibits production of TNF-α, IL-1β and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes. Rheumatology (Oxford) 45, 703–710 (2006).
Wozniacka, A. et al. The influence of antimalarial treatment on IL-1β, IL-6 and TNF-α mRNA expression on UVB-irradiated skin in systemic lupus erythematosus. Br. J. Dermatol. 159, 1124–1130 (2008).
Napirei, M. et al. Features of systemic lupus erythematosus in DNase1-deficient mice. Nat. Genet. 25, 177–181 (2000).
Chitrabamrung, S., Rubin, R. L. & Tan, E. M. Serum deoxyribonuclease I and clinical activity in systemic lupus erythematosus. Rheumatol. Int. 1, 55–60 (1981).
Marshak-Rothstein, A. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 6, 823–835 (2006).
Blasius, A. L. & Beutler, B. Intracellular Toll-like receptors. Immunity 32, 305–315 (2010).
Vallin, H., Blomberg, S., Alm, G. V., Cederblad, B. & Ronnblom, L. Patients with systemic lupus erythematosus (SLE) have a circulating inducer of interferon-α (IFN-α) production acting on leucocytes resembling immature dendritic cells. Clin. Exp. Immunol. 115, 196–202 (1999).
Ronnblom, L. & Alm, G. V. The natural interferon-α producing cells in systemic lupus erythematosus. Hum. Immunol. 63, 1181–1193 (2002).
Garcia-Romo, G. S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20. (2011).
Bird, A. P., Taggart, M. H., Nicholls, R. D. & Higgs, D. R. Non-methylated CpG-rich islands at the human α-globin locus: implications for evolution of the α-globin pseudogene. Embo J. 6, 999–1004 (1987).
Cornelie, S. et al. Direct evidence that Toll-like receptor 9 (TLR9) functionally binds plasmid DNA by specific cytosine–phosphate–guanine motif recognition. J. Biol. Chem. 279, 15124–15129 (2004).
Hong, Z. et al. Chloroquine protects mice from challenge with CpG ODN and LPS by decreasing proinflammatory cytokine release. Int. Immunopharmacol. 4, 223–234 (2004).
Macfarlane, D. E. & Manzel, L. Antagonism of immunostimulatory CpG-oligodeoxynucleotides by quinacrine, chloroquine, and structurally related compounds. J. Immunol. 160, 1122–1131 (1998).
Vollmer, J. et al. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J. Exp. Med. 202, 1575–1585 (2005).
de Bouteiller, O. et al. Recognition of double-stranded RNA by human Toll-like receptor 3 and downstream receptor signaling requires multimerization and an acidic pH. J. Biol. Chem. 280, 38133–38145 (2005).
Ewald, S. E. et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature 456, 658–662 (2008).
Kuznik, A. et al. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J. Immunol. 186, 4794–4804 (2011).
Furukawa, F., Kashihara-Sawami, M., Lyons, M. B. & Norris, D. A. Binding of antibodies to the extractable nuclear antigens SS-A/Ro and SS-B/La is induced on the surface of human keratinocytes by ultraviolet light (UVL): implications for the pathogenesis of photosensitive cutaneous lupus. J. Invest. Dermatol. 94, 77–85 (1990).
Shaffer, B., Cahn, M. M. & Levy, E. J. Absorption of antimalarial drugs in human skin; spectroscopic and chemical analysis in epidermis and corium. J. Invest. Dermatol. 30, 341–345 (1958).
Nguyen, T. Q., Capra, J. D. & Sontheimer, R. D. 4-aminoquinoline antimalarials enhance UV-B induced c-Jun transcriptional activation. Lupus 7, 148–153 (1998).
Wallace, D. J., Metzger, A. L., Stecher, V. J., Turnbull, B. A. & Kern, P. A. Cholesterol-lowering effect of hydroxychloroquine in patients with rheumatic disease: reversal of deleterious effects of steroids on lipids. Am. J. Med. 89, 322–326 (1990).
Goldstein, J. L., Brunschede, G. Y. & Brown, M. S. Inhibition of proteolytic degradation of low density lipoprotein in human fibroblasts by chloroquine, concanavalin A, and Triton WR 1339. J. Biol. Chem. 250, 7854–7862 (1975).
Lange, Y., Duan, H. & Mazzone, T. Cholesterol homeostasis is modulated by amphiphiles at transcriptional and post-transcriptional loci. J. Lipid Res. 37, 534–539 (1996).
Gu, J. Q. et al. A Toll-like receptor 9-mediated pathway stimulates perilipin 3 (TIP47) expression and induces lipid accumulation in macrophages. Am. J. Physiol. Endocrinol. Metab. 299, E593–E600 (2010).
Tobias, P. & Curtiss, L. K. Thematic review series: the immune system and atherogenesis. Paying the price for pathogen protection: Toll receptors in atherogenesis. J. Lipid Res. 46, 404–411 (2005).
Lesiak, A. et al. Systematic administration of chloroquine in discoid lupus erythematosus reduces skin lesions via inhibition of angiogenesis. Clin. Exp. Dermatol. 34, 570–575 (2009).
Pinhal-Enfield, G. et al. An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A2A receptors. Am. J. Pathol. 163, 711–721 (2003).
Espinola, R. G., Pierangeli, S. S., Gharavi, A. E. & Harris, E. N. Hydroxychloroquine reverses platelet activation induced by human IgG antiphospholipid antibodies. Thromb. Haemost. 87, 518–522 (2002).
Rand, J. H. et al. Hydroxychloroquine protects the annexin A5 anticoagulant shield from disruption by antiphospholipid antibodies: evidence for a novel effect for an old antimalarial drug. Blood 115, 2292–2299 (2010).
Woessner, J. F. Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. Faseb J. 5, 2145–2154 (1991).
Stuhlmeier, K. M. & Pollaschek, C. Quinacrine but not chloroquine inhibits PMA induced upregulation of matrix metalloproteinases in leukocytes: quinacrine acts at the transcriptional level through a PLA2-independent mechanism. J. Rheumatol. 33, 472–480 (2006).
Lesiak, A. et al. Effect of chloroquine phosphate treatment on serum MMP-9 and TIMP-1 levels in patients with systemic lupus erythematosus. Lupus 19, 683–688 (2010).
Merrell, M. A. et al. Toll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activity. Mol. Cancer Res. 4, 437–447 (2006).
Lullmann-Rauch, R., Pods, R. & von Witzendorff, B. The antimalarials quinacrine and chloroquine induce weak lysosomal storage of sulphated glycosaminoglycans in cell culture and in vivo. Toxicology 110, 27–37 (1996).
Toubi, E. et al. The reduction of serum B-lymphocyte activating factor levels following quinacrine add-on therapy in systemic lupus erythematosus. Scand. J. Immunol. 63, 299–303 (2006).
Ehsanian, R., Van Waes, C. & Feller, S. M. Beyond DNA binding—a review of the potential mechanisms mediating quinacrine's therapeutic activities in parasitic infections, inflammation, and cancers. Cell Commun. Signal. 9, 13 (2011).
[No authors listed] A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. The Canadian Hydroxychloroquine Study Group. N. Engl. J. Med. 324, 150–154 (1991).
Molad, Y. et al. Protective effect of hydroxychloroquine in systemic lupus erythematosus. Prospective long-term study of an Israeli cohort. Lupus 11, 356–361 (2002).
Fessler, B. J. et al. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum. 52, 1473–1480 (2005).
Alarcon, G. S. et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann. Rheum. Dis. 66, 1168–1172 (2007).
Ruiz-Irastorza, G. et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus 15, 577–583 (2006).
James, J. A. et al. Hydroxychloroquine sulfate treatment is associated with later onset of systemic lupus erythematosus. Lupus 16, 401–409 (2007).
Shinjo, S. K. et al. Antimalarial treatment may have a time-dependent effect on lupus survival: data from a multinational Latin American inception cohort. Arthritis Rheum. 62, 855–862 (2010).
Pons-Estel, G. J. et al. Possible protective effect of hydroxychloroquine on delaying the occurrence of integument damage in lupus: LXXI, data from a multiethnic cohort. Arthritis Care Res. (Hoboken) 62, 393–400 (2010).
Wallace, D. J. Does hydroxychloroquine sulfate prevent clot formation in systemic lupus erythematosus? Arthritis Rheum. 30, 1435–1436 (1987).
Ho, K. T. et al. Systemic lupus erythematosus in a multiethnic cohort (LUMINA): XXVIII. Factors predictive of thrombotic events. Rheumatology (Oxford) 44, 1303–1307 (2005).
Sisó, A. et al. Previous antimalarial therapy in patients diagnosed with lupus nephritis: influence on outcomes and survival. Lupus 17, 281–288 (2008).
Kaiser, R., Cleveland, C. M. & Criswell, L. A. Risk and protective factors for thrombosis in systemic lupus erythematosus: results from a large, multi-ethnic cohort. Ann. Rheum. Dis. 68, 238–241 (2009).
Tektonidou, M. G., Laskari, K., Panagiotakos, D. B. & Moutsopoulos, H. M. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Rheum. 61, 29–36 (2009).
Jung, H. et al. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum. 62, 863–868 (2010).
Petri, M., Lakatta, C., Magder, L. & Goldman, D. Effect of prednisone and hydroxychloroquine on coronary artery disease risk factors in systemic lupus erythematosus: a longitudinal data analysis. Am. J. Med. 96, 254–259 (1994).
Kavanaugh, A., Adams-Huet, B., Jain, R., Denke, M. & McFarlin, J. Hydroxychloroquine effects on lipoprotein profiles (the HELP trial): a double-blind, randomized, placebo-controlled, pilot study in patients with systemic lupus erythematosus. J. Clin. Rheumatol. 3, 3–8 (1997).
Borba, E. F. & Bonfa, E. Longterm beneficial effect of chloroquine diphosphate on lipoprotein profile in lupus patients with and without steroid therapy. J. Rheumatol. 28, 780–785 (2001).
Petri, M. Hydroxychloroquine use in the Baltimore Lupus Cohort: effects on lipids, glucose and thrombosis. Lupus 5 (Suppl. 1), S16–S22 (1996).
Pons-Estel, G. J. et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum. 61, 830–839 (2009).
Pons-Estel, G. J. et al. Anti-malarials exert a protective effect while Mestizo patients are at increased risk of developing SLE renal disease: data from a Latin-American cohort. Rheumatology (Oxford) http://dx.doi.org/10.1093/rheumatology/ker514.
Ruiz-Irastorza, G. et al. Predictors of major infections in systemic lupus erythematosus. Arthritis Res. Ther. 11, R109 (2009).
Ruiz-Irastorza, G., Ramos-Casals, M., Brito-Zeron, P. & Khamashta, M. A. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann. Rheum. Dis. 69, 20–28 (2010).
Schmajuk, G., Yazdany, J., Trupin, L. & Yelin, E. Hydroxychloroquine treatment in a community-based cohort of patients with systemic lupus erythematosus. Arthritis Care Res. (Hoboken) 62, 386–392 (2010).
Ruzicka, T., Sommerburg, C., Goerz, G., Kind, P. & Mensing, H. Treatment of cutaneous lupus erythematosus with acitretin and hydroxychloroquine. Br. J. Dermatol. 127, 513–518 (1992).
Cavazzana, I. et al. Treatment of lupus skin involvement with quinacrine and hydroxychloroquine. Lupus 18, 735–739 (2009).
Chang, A. Y. et al. Response to antimalarial agents in cutaneous lupus erythematosus: a prospective analysis. Arch. Dermatol. 147, 1261–1267 (2011).
Rahman, P., Gladman, D. D. & Urowitz, M. B. Smoking interferes with efficacy of antimalarial therapy in cutaneous lupus. J. Rheumatol. 25, 1716–1719 (1998).
Jewell, M. L. & McCauliffe, D. P. Patients with cutaneous lupus erythematosus who smoke are less responsive to antimalarial treatment. J. Am. Acad. Dermatol. 42, 983–987 (2000).
Kreuter, A. et al. Lupus erythematosus tumidus: response to antimalarial treatment in 36 patients with emphasis on smoking. Arch. Dermatol. 145, 244–248 (2009).
Leroux, G. et al. Relationship between blood hydroxychloroquine and desethylchloroquine concentrations and cigarette smoking in treated patients with connective tissue diseases. Ann. Rheum. Dis. 66, 1547–1548 (2007).
Wright, J. L., Tai, H., Wang, R., Wang, X. & Churg, A. Cigarette smoke upregulates pulmonary vascular matrix metalloproteinases via TNF-α signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L125–L133 (2007).
Lim, S. et al. Balance of matrix metalloprotease-9 and tissue inhibitor of metalloprotease-1 from alveolar macrophages in cigarette smokers. Regulation by interleukin-10. Am. J. Respir. Crit. Care Med. 162, 1355–1360 (2000).
Bermudez, E. A., Rifai, N., Buring, J. E., Manson, J. E. & Ridker, P. M. Relation between markers of systemic vascular inflammation and smoking in women. Am. J. Cardiol. 89, 1117–1119 (2002).
Lardet, D. et al. Effect of smoking on the effectiveness of antimalarial drugs for cutaneous lesions of patients with lupus: assessment in a prospective study [French]. Rev. Med. Interne 25, 786–791 (2004).
Wahie, S. et al. Clinical and pharmacogenetic influences on response to hydroxychloroquine in discoid lupus erythematosus: a retrospective cohort study. J. Invest. Dermatol. 131, 1981–1986 (2011).
Turchin, I., Bernatsky, S., Clarke, A. E., St-Pierre, Y. & Pineau, C. A. Cigarette smoking and cutaneous damage in systemic lupus erythematosus. J. Rheumatol. 36, 2691–2693 (2009).
Moghadam-Kia, S. et al. Cross-sectional analysis of a collaborative Web-based database for lupus erythematosus-associated skin lesions: prospective enrollment of 114 patients. Arch. Dermatol. 145, 255–260 (2009).
Piette, E. W. et al. Impact of smoking in cutaneous lupus erythematosus. Arch. Dermatol. 148, 317–322 (2012).
Parke, A. Antimalarial drugs and pregnancy. Am. J. Med. 85, 30–33 (1988).
Parke, A. & West, B. Hydroxychloroquine in pregnant patients with systemic lupus erythematosus. J. Rheumatol. 23, 1715–1718 (1996).
Buchanan, N. M. et al. Hydroxychloroquine and lupus pregnancy: review of a series of 36 cases. Ann. Rheum. Dis. 55, 486–488 (1996).
Clowse, M. E., Magder, L., Witter, F. & Petri, M. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum. 54, 3640–3647 (2006).
Izmirly, P. M. et al. Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine. Ann. Rheum. Dis. 69, 1827–1830 (2010).
Levy, R. A. et al. Hydroxychloroquine (HCQ) in lupus pregnancy: double-blind and placebo-controlled study. Lupus 10, 401–404 (2001).
Costedoat-Chalumeau, N., Amoura, Z., Huong, D. L., Lechat, P. & Piette, J. C. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases. Review of the literature. Autoimmun. Rev. 4, 111–115 (2005).
Wallace, D. J. in Dubois Lupus Erythematosus 7th edn (eds Wallace, D. J. & Hahn, B. H.) 1152–1176 (Lippincott Williams & Wilkins, Philadelphia, 2007).
Van Beek, M. J. & Piette, W. W. Antimalarials. Dermatol. Clin. 19, 147–160, ix (2001).
Marmor, M. F., Kellner, U., Lai, T. Y., Lyons, J. S. & Mieler, W. F. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 118, 415–422 (2011).
Venuturupalli, S. R., Gudsoorkar, V. S. & Wallace, D. J. Revisiting antimalarials in systemic lupus erythematosus: developments of translational clinical interest. J. Rheumatol. (in press).
Wallace, D. J. Advances in drug therapy for systemic lupus erythematosus. BMC Med. 8, 77 (2010).
Wozniacka, A., Carter, A. & McCauliffe, D. P. Antimalarials in cutaneous lupus erythematosus: mechanisms of therapeutic benefit. Lupus 11, 71–81 (2002).
Lopez, P., Gomez, J., Mozo, L., Gutierrez, C. & Suarez, A. Cytokine polymorphisms influence treatment outcomes in SLE patients treated with antimalarial drugs. Arthritis Res. Ther. 8, R42 (2006).
Collinge, J. et al. Safety and efficacy of quinacrine in human prion disease (PRION-1 study): a patient-preference trial. Lancet Neurol. 8, 334–344 (2009).
Gurova, K. New hopes from old drugs: revisiting DNA-binding small molecules as anticancer agents. Future Oncol. 5, 1685–1704 (2009).
Adelusi, S. A. & Salako, L. A. Tissue and blood concentrations of chloroquine following chronic administration in the rat. J. Pharm. Pharmacol. 34, 733–735 (1982).
Ronnblom, L., Eloranta, M. L. & Alm, G. V. The type I interferon system in systemic lupus erythematosus. Arthritis Rheum. 54, 408–420 (2006).
Aman, M. J. et al. Interferon-α stimulates production of interleukin-10 in activated CD4+ T cells and monocytes. Blood 87, 4731–4736 (1996).
Ronnblom, L., Alm, G. V. & Eloranta, M. L. The type I interferon system in the development of lupus. Semin. Immunol. 23, 113–121 (2011).
Villanueva, E. et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187, 538–552 (2011).
Author information
Authors and Affiliations
Contributions
D. J. Wallace, V. S. Gudsoorkar and S. R. Venuturupalli researched the data for the article. All authors provided a substantial contribution to discussions of the content and contributed equally to writing the article and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wallace, D., Gudsoorkar, V., Weisman, M. et al. New insights into mechanisms of therapeutic effects of antimalarial agents in SLE. Nat Rev Rheumatol 8, 522–533 (2012). https://doi.org/10.1038/nrrheum.2012.106
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2012.106
This article is cited by
-
Neuroprotective role of chloroquine via modulation of autophagy and neuroinflammation in MPTP-induced Parkinson’s disease
Inflammopharmacology (2023)
-
Identification of the potential TLR7 antagonists by virtual screening and experimental validation
Molecular Diversity (2023)
-
Hydroxychloroquine induces apoptosis of myeloid-derived suppressor cells via up-regulation of CD81 contributing to alleviate lupus symptoms
Molecular Medicine (2022)
-
Hydroxychloroquine non-availability during COVID-19 pandemic and its relation to anxiety level and disease activity in rheumatoid arthritis and lupus patients: a cross-sectional study
Egyptian Rheumatology and Rehabilitation (2022)
-
Antidiabetic effects of hydroxychloroquine in two Japanese patients with systemic lupus erythematosus
Diabetology International (2022)