Abstract
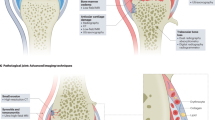
New MRI techniques have been developed to assess not only the static anatomy of synovial hyperplasia, bone changes and cartilage degradation in patients with rheumatoid arthritis (RA), but also the activity of the physiological events that cause these changes. This enables an estimation of the rate of change in the synovium, bone and cartilage as a result of disease activity or in response to therapy. Typical MRI signs of RA in the pre-erosive phase include synovitis, bone marrow edema and subchondral cyst formation. Synovitis can be assessed by T2-weighted imaging, dynamic contrast-enhanced MRI or diffusion tensor imaging. Bone marrow edema can be detected on fluid-sensitive sequences such as short-tau inversion recovery or T2-weighted fast-spin echo sequences. Detection of small bone erosions in the early erosive phase using T1-weighted MRI has sensitivity comparable to CT. Numerous MRI techniques have been developed for quantitative assessment of potentially pathologic changes in cartilage composition that occur before frank morphologic changes. In this Review, we summarize the advances and new directions in the field of MRI, with an emphasis on their current state of development and application in RA.
Key Points
-
The optimal roles of MRI in rheumatoid arthritis (RA) research and clinical practice lie in identifying the early, pre-erosive stages of disease and monitoring therapy
-
MRI signs of synovitis have been validated histologically and clinically both in animal studies and in humans
-
Modern high-field extremity MRI scanners maximize patient comfort without sacrificing image quality, and have been validated against conventional MRI units using the RAMRIS (rheumatoid arthritis magnetic resonance imaging score) system
-
The capability of MRI to quantify outcome measures provides a powerful research tool that can also incorporate pharmacokinetic modeling
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Taylor, P. C. & Feldmann, M. Anti-TNF biologic agents: still the therapy of choice for rheumatoid arthritis. Nat. Rev. Rheumatol. 5, 578–582 (2009).
Maini, R. N. et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 54, 2817–2829 (2006).
Cohen, S. B. et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 58, 1299–1309 (2008).
Lewiecki, E. M. Denosumab update. Curr. Opin. Rheumatol. 21, 369–373 (2009).
van den Berg, W. B. & Miossec, P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat. Rev. Rheumatol. 5, 549–553 (2009).
Kremer, J. M. et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 60, 1895–1905 (2009).
Dorner, T. & Burmester, G. R. New approaches of B-cell-directed therapy: beyond rituximab. Curr. Opin. Rheumatol. 20, 263–268 (2008).
Dorner, T., Radbruch, A. & Burmester, G. R. B-cell-directed therapies for autoimmune disease. Nat. Rev. Rheumatol. 5, 433–441 (2009).
Eisenberg, R. & Albert, D. B-cell targeted therapies in rheumatoid arthritis and systemic lupus erythematosus. Nat. Clin. Pract. Rheumatol. 2, 20–27 (2006).
Steinberg, J. J., Kincaid, S. B. & Sledge, C. B. Inhibition of cartilage breakdown by hydrocortisone in a tissue culture model of rheumatoid arthritis. Ann. Rheum. Dis. 42, 323–330 (1983).
Arner, E. C., Harris, R. R., DiMeo, T. M., Collins, R. C. & Galbraith, W. Interleukin-1 receptor antagonist inhibits proteoglycan breakdown in antigen induced but not polycation induced arthritis in the rabbit. J. Rheumatol. 22, 1338–1346 (1995).
Yasuda, T. & Poole, A. R. A fibronectin fragment induces type II collagen degradation by collagenase through an interleukin-1-mediated pathway. Arthritis Rheum. 46, 138–148 (2002).
Yodlowski, M. L. et al. Antibody to interleukin 1 inhibits the cartilage degradative and thymocyte proliferative actions of rheumatoid synovial culture medium. J. Rheumatol. 17, 1600–1607 (1990).
Elliott, S. & Cawston, T. The clinical potential of matrix metalloproteinase inhibitors in the rheumatic disorders. Drugs Aging 18, 87–99 (2001).
Nagase, H. & Kashiwagi, M. Aggrecanases and cartilage matrix degradation. Arthritis Res. Ther. 5, 94–103 (2003).
Medicherla, S. et al. A selective p38 alpha mitogen-activated protein kinase inhibitor reverses cartilage and bone destruction in mice with collagen-induced arthritis. J. Pharmacol. Exp. Ther. 318, 132–141 (2006).
Catrina, A. I. et al. Anti-tumour necrosis factor (TNF)-alpha therapy (etanercept) down-regulates serum matrix metalloproteinase (MMP)-3 and MMP-1 in rheumatoid arthritis. Rheumatology (Oxford) 41, 484–489 (2002).
Ranganathan, P. An update on pharmacogenomics in rheumatoid arthritis with a focus on TNF-blocking agents. Curr. Opin. Mol. Ther. 10, 562–567 (2008).
Moran, E. M. et al. Human rheumatoid arthritis tissue production of IL-17A drives matrix and cartilage degradation: synergy with tumour necrosis factor-alpha, Oncostatin M and response to biologic therapies. Arthritis Res. Ther. 11, R113 (2009).
Rannou, F., Francois, M., Corvol, M. T. & Berenbaum, F. Cartilage breakdown in rheumatoid arthritis. Joint Bone Spine 73, 29–36 (2006).
McQueen, F. et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Summary of OMERACT 6 MR imaging module. J. Rheumatol. 30, 1387–1392 (2003).
McQueen, F. M. et al. Bone edema scored on magnetic resonance imaging scans of the dominant carpus at presentation predicts radiographic joint damage of the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum. 48, 1814–1827 (2003).
Schirmer, C. et al. Diagnostic quality and scoring of synovitis, tenosynovitis and erosions in low-field MRI of patients with rheumatoid arthritis: a comparison with conventional MRI. Ann. Rheum. Dis. 66, 522–529 (2007).
Østergaard, M. et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J. Rheumatol. 30, 1385–1386 (2003).
Haavardsholm, E. A. et al. Reliability and sensitivity to change of the OMERACT rheumatoid arthritis magnetic resonance imaging score in a multireader, longitudinal setting. Arthritis Rheum. 52, 3860–3867 (2005).
Bird, P. et al. A multireader reliability study comparing conventional high-field magnetic resonance imaging with extremity low-field MRI in rheumatoid arthritis. J. Rheumatol. 34, 854–856 (2007).
Conaghan, P. G. et al. A multicenter reliability study of extremity-magnetic resonance imaging in the longitudinal evaluation of rheumatoid arthritis. J. Rheumatol. 34, 857–858 (2007).
Klarlund, M. et al. Magnetic resonance imaging, radiography, and scintigraphy of the finger joints: one year follow up of patients with early arthritis. Ann. Rheum. Dis. 59, 521–528 (2000).
McQueen, F. M. et al. What is the fate of erosions in early rheumatoid arthritis? Tracking individual lesions using x rays and magnetic resonance imaging over the first two years of disease. Ann. Rheum. Dis. 60, 859–868 (2001).
McQueen, F. M. et al. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals progression of erosions despite clinical improvement. Ann. Rheum. Dis. 58, 156–163 (1999).
Jevtic, V. et al. Precontrast and postcontrast (Gd-DTPA) magnetic resonance imaging of hand joints in patients with rheumatoid arthritis. Clin. Radiol. 48, 176–181 (1993).
Brennan, P. et al. A simple algorithm to predict the development of radiological erosions in patients with early rheumatoid arthritis: prospective cohort study. BMJ 313, 471–476 (1996).
Sugimoto, H., Takeda, A. & Hyodoh, K. Early-stage rheumatoid arthritis: prospective study of the effectiveness of MR imaging for diagnosis. Radiology 216, 569–575 (2000).
Sommer, O. J. et al. Rheumatoid arthritis: a practical guide to state-of-the-art imaging, image interpretation, and clinical implications. Radiographics 25, 381–398 (2005).
Ostendorf, B. et al. Magnetic resonance imaging and miniarthroscopy of metacarpophalangeal joints: Sensitive detection of morphologic changes in rheumatoid arthritis. Arthritis Rheum. 44, 2492–2502 (2001).
Ejbjerg, B. J., Narvestad, E., Jacobsen, S., Thomsen, H. S. & Østergaard, M. Optimised, low cost, low field dedicated extremity MRI is highly specific and sensitive for synovitis and bone erosions in rheumatoid arthritis wrist and finger joints: comparison with conventional high field MRI and radiography. Ann. Rheum. Dis. 64, 1280–1287 (2005).
Hetland, M. L. et al. MRI bone oedema is the strongest predictor of subsequent radiographic progression in early rheumatoid arthritis. Results from a 2-year randomised controlled trial (CIMESTRA). Ann. Rheum. Dis. 68, 384–390 (2009).
Naraghi, A. M., White, L. M., Patel, C., Tomlinson, G. & Keystone, E. C. Comparison of 1.0-T extremity MR and 1.5-T conventional high-field-strength MR in patients with rheumatoid arthritis. Radiology 251, 829–837 (2009).
Tehranzadeh, J., Ashikyan, O. & Dascalos, J. Advanced imaging of early rheumatoid arthritis. Radiol. Clin. North Am. 42, 89–107 (2004).
Shabana, W. M. et al. Nephrogenic systemic fibrosis: a report of 29 cases. AJR Am. J. Roentgenol. 190, 736–741 (2008).
Marckmann, P. et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J. Am. Soc. Nephrol. 17, 2359–2362 (2006).
Ostergaard, M. et al. Reducing invasiveness, duration, and cost of magnetic resonance imaging in rheumatoid arthritis by omitting intravenous contrast injection—does it change the assessment of inflammatory and destructive joint changes by the OMERACT RAMRIS? J. Rheumatol. 36, 1806–1810 (2009).
Narváez, J. A., Narváez, J., De Lama, E. & De Albert, M. MR imaging of early rheumatoid arthritis. Radiographics 30, 143–163 (2010).
Narváez, J. A., Narváez, J., Roca, Y. & Aguilera, C. MR imaging assessment of clinical problems in rheumatoid arthritis. Eur. Radiol. 12, 1819–1828 (2002).
Ostergaard, M. & Ejbjerg, B. Magnetic resonance imaging of the synovium in rheumatoid arthritis. Semin. Musculoskelet. Radiol. 8, 287–299 (2004).
Rand, T. et al. Discrimination between fluid, synovium, and cartilage in patients with rheumatoid arthritis: contrast enhanced spin echo versus non-contrast-enhanced fat-suppressed gradient echo MR imaging. Clin. Radiol. 54, 107–110 (1999).
van der Leij, C., van de Sande, M. G., Lavini, C., Tak, P. P. & Maas, M. Rheumatoid synovial inflammation: pixel-by-pixel dynamic contrast-enhanced MR imaging time-intensity curve shape analysis—a feasibility study. Radiology 253, 234–240 (2009).
Hodgson, R. et al. Dynamic contrast enhanced MRI of bone marrow oedema in rheumatoid arthritis. Ann. Rheum. Dis. 67, 270–272 (2008).
Hodgson, R. J. et al. Changes underlying the dynamic contrast-enhanced MRI response to treatment in rheumatoid arthritis. Skeletal Radiol. 37, 201–207 (2008).
Zierhut, M. L., Gardner, J. C., Spilker, M. E., Sharp, J. T. & Vicini, P. Kinetic modeling of contrast-enhanced MRI: an automated technique for assessing inflammation in the rheumatoid arthritis wrist. Ann. Biomed. Eng. 35, 781–795 (2007).
Boesen, M. et al. MRI quantification of rheumatoid arthritis: current knowledge and future perspectives. Eur. J. Radiol. 71, 189–196 (2009).
Kubassova, O., Boesen, M., Cimmino, M. A. & Bliddal, H. A computer-aided detection system for rheumatoid arthritis MRI data interpretation and quantification of synovial activity. Eur. J. Radiol. 74, e67–e72 (2010).
Peloschek, P. et al. Assessement of rheumatic diseases with computational radiology: current status and future potential. Eur. J. Radiol. 71, 211–216 (2009).
Ostergaard, M. et al. Quantification of synovistis by MRI: correlation between dynamic and static gadolinium-enhanced magnetic resonance imaging and microscopic and macroscopic signs of synovial inflammation. Magn. Reson. Imaging 16, 743–754 (1998).
Cimmino, M. A. et al. Dynamic gadolinium-enhanced magnetic resonance imaging of the wrist in patients with rheumatoid arthritis can discriminate active from inactive disease. Arthritis Rheum. 48, 1207–1213 (2003).
Agarwal, V. et al. Diffusion tensor anisotropy magnetic resonance imaging: a new tool to assess synovial inflammation. Rheumatology (Oxford) 48, 378–382 (2009).
Dalbeth, N. et al. Cellular characterisation of magnetic resonance imaging bone oedema in rheumatoid arthritis; implications for pathogenesis of erosive disease. Ann. Rheum. Dis. 68, 279–282 (2009).
Hashizume, M., Hayakawa, N. & Mihara, M. IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17. Rheumatology (Oxford) 47, 1635–1640 (2008).
Li, X. et al. Quantitative assessment of bone marrow edema-like lesion and overlying cartilage in knees with osteoarthritis and anterior cruciate ligament tear using MR imaging and spectroscopic imaging at 3 Tesla. J. Magn. Reson. Imaging 28, 453–461 (2008).
Mayerhoefer, M. E. et al. Computer-assisted quantitative analysis of bone marrow edema of the knee: initial experience with a new method. AJR Am. J. Roentgenol. 182, 1399–1403 (2004).
Brittberg, M. & Winalski, C. S. Evaluation of cartilage injuries and repair. J. Bone Joint Surg. Am. 85, 58–69 (2003).
McQueen, F. M. et al. High-grade MRI bone oedema is common within the surgical field in rheumatoid arthritis patients undergoing joint replacement and is associated with osteitis in subchondral bone. Ann. Rheum. Dis. 66, 1581–1587 (2007).
Bugatti, S. et al. Involvement of subchondral bone marrow in rheumatoid arthritis: lymphoid neogenesis and in situ relationship to subchondral bone marrow osteoclast recruitment. Arthritis Rheum. 52, 3448–3459 (2005).
Dohn, U. M. et al. Are bone erosions detected by magnetic resonance imaging and ultrasonography true erosions? A comparison with computed tomography in rheumatoid arthritis metacarpophalangeal joints. Arthritis Res. Ther. 8, R110 (2006).
Perry, D. et al. Detection of erosions in the rheumatoid hand; a comparative study of multidetector computerized tomography versus magnetic resonance scanning. J. Rheumatol. 32, 256–267 (2005).
Van Breuseghem, I. Ultrastructural MR imaging techniques of the knee articular cartilage: problems for routine clinical application. Eur. Radiol. 14, 184–192 (2004).
Billinghurst, R. C. et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J. Clin. Invest. 99, 1534–1545 (1997).
Buckwalter, J. A. & Mankin, H. J. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr. Course Lect. 47, 477–486 (1998).
Andreas, K. et al. Key regulatory molecules of cartilage destruction in rheumatoid arthritis: an in vitro study. Arthritis Res. Ther. 10, R9 (2008).
Abramson, S. B., Attur, M. & Yazici, Y. Prospects for disease modification in osteoarthritis. Nat. Clin. Pract. Rheumatol. 2, 304–312 (2006).
Fragonas, E. et al. Correlation between biochemical composition and magnetic resonance appearance of articular cartilage. Osteoarthritis Cartilage 6, 24–32 (1998).
Smith, H. E. et al. Spatial variation in cartilage T2 of the knee. J. Magn. Reson. Imaging 14, 50–55 (2001).
Mosher, T. J., Dardzinski, B. J. & Smith, M. B. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2--preliminary findings at 3 T. Radiology 214, 259–266 (2000).
Xia, Y., Moody, J. B. & Alhadlaq, H. Orientational dependence of T2 relaxation in articular cartilage: a microscopic MRI (microMRI) study. Magn. Reson. Med. 48, 460–469 (2002).
Dardzinski, B. J., Mosher, T. J., Li, S., Van Slyke, M. A. & Smith, M. B. Spatial variation of T2 in human articular cartilage. Radiology 205, 546–550 (1997).
Potter, K., Butler, J. J., Horton, W. E. & Spencer, R. G. Response of engineered cartilage tissue to biochemical agents as studied by proton magnetic resonance microscopy. Arthritis Rheum. 43, 1580–1590 (2000).
Nieminen, M. T. et al. Quantitative MR microscopy of enzymatically degraded articular cartilage. Magn. Reson. Med. 43, 676–681 (2000).
Kight, A. C., Dardzinski, B. J., Laor, T. & Graham, T. B. Magnetic resonance imaging evaluation of the effects of juvenile rheumatoid arthritis on distal femoral weight-bearing cartilage. Arthritis Rheum. 50, 901–905 (2004).
Bashir, A., Gray, M. L. & Burstein, D. Gd-DTPA2 as a measure of cartilage degradation. Magn. Reson. Med. 36, 665–673 (1996).
Bashir, A., Gray, M. L., Boutin, R. D. & Burstein, D. Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)(2-)-enhanced MR imaging. Radiology 205, 551–558 (1997).
Nieminen, M. T. et al. Spatial assessment of articular cartilage proteoglycans with Gd-DTPA-enhanced T1 imaging. Magn. Reson. Med. 48, 640–648 (2002).
Sur, S., Mamisch, T. C., Hughes, T. & Kim, Y. J. High resolution fast T1 mapping technique for dGEMRIC. J. Magn. Reson. Imaging 30, 896–900 (2009).
Reddy, R. et al. Sodium MRI of human articular cartilage in vivo. Magn. Reson. Med. 39, 697–701 (1998).
Koff, M. F. & Potter, H. G. Noncontrast MR techniques and imaging of cartilage. Radiol. Clin. North Am. 47, 495–504 (2009).
Regatte, R. R., Akella, S. V., Borthakur, A., Kneeland, J. B. & Reddy, R. In vivo proton MR three-dimensional T1ρ mapping of human articular cartilage: initial experience. Radiology 229, 269–274 (2003).
Li, X. et al. In vivo T1ρ and T2 mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage 15, 789–797 (2007).
Li, X. et al. In vivo 3 T spiral imaging based multi-slice T1ρ mapping of knee cartilage in osteoarthritis. Magn. Reson. Med. 54, 929–936 (2005).
Akella, S. V. et al. Proteoglycan-induced changes in T1ρ-relaxation of articular cartilage at 4 T. Magn. Reson. Med. 46, 419–423 (2001).
Dohn, U. M. et al. Detection of bone erosions in rheumatoid arthritis wrist joints with magnetic resonance imaging, computed tomography and radiography. Arthritis Res. Ther. 10, R25 (2008).
Borthakur, A. et al. Quantifying sodium in the human wrist in vivo by using MR imaging. Radiology 224, 598–602 (2002).
Acknowledgements
Funding for this research was made possible by grants from the ACR Research and Education Foundation Within Our Reach: Finding a Cure for Rheumatoid Arthritis campaign, the Alliance for Lupus Research—Target Identification in Lupus program, VA Merit Review Grants (1I01BX000600-01), Daiichi-Sankyo Co., Ltd., and NIH grants (1AI 071110 and ARRA 3RO1AI71110-02S1) (all to J. D. Mountz). We also thank Dr Hui-Chen Hsu for critical reading of this manuscript and Dr Fiona Hunter for editorial suggestions. We also thank Ms Carol Humber and Ms Darlene M. Frasher for assistance with manuscript preparation.
Author information
Authors and Affiliations
Contributions
C. G. Borrero and J. D. Mountz researched data for the article and made substantial contributions to discussion of the content. C. G. Borrero wrote the article. C. G. Borrero and J. M. Mountz performed reviewing/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Borrero, C., Mountz, J. & Mountz, J. Emerging MRI methods in rheumatoid arthritis. Nat Rev Rheumatol 7, 85–95 (2011). https://doi.org/10.1038/nrrheum.2010.173
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2010.173
This article is cited by
-
The role of advanced MRI in the development of treat-to-target therapeutic strategies, patient stratification and phenotyping in rheumatoid arthritis
BMC Rheumatology (2020)
-
miR-449a inhibits cell proliferation, migration, and inflammation by regulating high-mobility group box protein 1 and forms a mutual inhibition loop with Yin Yang 1 in rheumatoid arthritis fibroblast-like synoviocytes
Arthritis Research & Therapy (2019)
-
Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies
Bone Research (2018)
-
Focal bone involvement in inflammatory arthritis: the role of IL17
Rheumatology International (2016)
-
MRI of the wrist in juvenile idiopathic arthritis: erosions or normal variants? A prospective case-control study
Pediatric Radiology (2013)