Abstract
Background:
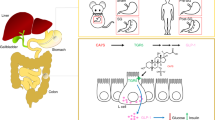
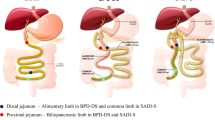
Bariatric surgery is currently the most efficacious treatment for obesity and its associated metabolic co-morbidities, such as diabetes. The metabolic improvements occur through both weight-dependent and weight-independent mechanisms. Bile acids (BAs) have emerged as key signalling molecules that have a central role in modulating many of the physiological effects seen after bariatric surgery. This systematic review assesses the evidence from both human and animal studies for the role of BAs in reducing the metabolic complications of obesity following bariatric surgery.
Methods:
We conducted a systematic search of Medline and Embase databases to identify all articles investigating the role of BAs in mediating the metabolic changes observed following bariatric surgery in both animal and human studies. Boolean logic was used with relevant search terms, including the following MeSH terms: ‘bile acids and salts’, ‘bariatric surgery’, ‘metabolic surgery’, ‘gastrointestinal tract/surgery’ and ‘obesity/surgery’.
Results:
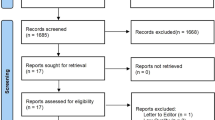
Following database searches (n=1197), inclusion from bibliography searches (n=2) and de-duplication (n=197), 1002 search results were returned. Of these, 132 articles were selected for full-text review, of which 38 articles were deemed relevant and included in the review. The findings support the effects of BAs on satiety, lipid and cholesterol metabolism, incretins and glucose homoeostasis, energy metabolism, gut microbiota and endoplasmic reticulum stress following bariatric surgery. Many of these metabolic effects are modulated through the BA receptors FXR and TGR5. We also explore a possible link between BAs and carcinogenesis following bariatric surgery.
Conclusions:
Overall there is good evidence to support the role of BAs in the metabolic effects of bariatric surgery through the above mechanisms. BAs could serve as a novel therapeutic pharmacological target for the treatment of obesity and its associated co-morbidities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med 2014; 370: 2002–2013.
Liang Z, Wu Q, Chen B, Yu P, Zhao H, Ouyang X . Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract 2013; 101: 50–56.
Dixon JB, Schachter LM, O'Brien PE, Jones K, Grima M, Lambert G et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA 2012; 308: 1142–1149.
Ashrafian H, Ahmed K, Rowland SP, Patel VM, Gooderham NJ, Holmes E et al. Metabolic surgery and cancer: protective effects of bariatric procedures. Cancer 2011; 117: 1788–1799.
Cummings BP, Graham JL, Stanhope KL, Chouinard ML, Havel PJ . Maternal ileal interposition surgery confers metabolic improvements to offspring independent of effects on maternal body weight in UCD-T2DM rats. Obes Surg 2013; 23: 2042–2049.
Ashrafian H, Bueter M, Ahmed K, Suliman A, Bloom SR, Darzi A et al. Metabolic surgery: an evolution through bariatric animal models. Obes Rev 2010; 11: 907–920.
Kohli R, Setchell KD, Kirby M, Myronovych A, Ryan KK, Ibrahim SH et al. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology 2013; 154: 2341–2351.
Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S . Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab 2013; 98: E708–E712.
Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009; 17: 1671–1677.
Ahmad NN, Pfalzer A, Kaplan LM . Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes (Lond) 2013; 37: 1553–1559.
Kohli R, Kirby M, Setchell KD, Jha P, Klustaitis K, Woollett LA et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol 2010; 299: G652–G660.
Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 2012; 153: 3613–3619.
Katona BW, Anant S, Covey DF, Stenson WF . Characterization of enantiomeric bile acid-induced apoptosis in colon cancer cell lines. J Biol Chem 2009; 284: 3354–3364.
Bernstein H, Bernstein C, Payne CM, Dvorak K . Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J Gastroenterol 2009; 15: 3329–3340.
Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA et al. Bile acids: natural ligands for an orphan nuclear receptor. Science 1999; 284: 1365–1368.
Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A et al. Identification of a nuclear receptor for bile acids. Science 1999; 284: 1362–1365.
Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 2000; 6: 517–526.
Brendel C, Schoonjans K, Botrugno OA, Treuter E, Auwerx J . The small heterodimer partner interacts with the liver X receptor alpha and represses its transcriptional activity. Mol Endocrinol 2002; 16: 2065–2076.
Zhang M, Chiang JY . Transcriptional regulation of the human sterol 12alpha-hydroxylase gene (CYP8B1): roles of heaptocyte nuclear factor 4alpha in mediating bile acid repression. J Biol Chem 2001; 276: 41690–41699.
Kir S, Zhang Y, Gerard RD, Kliewer SA, Mangelsdorf DJ . Nuclear receptors HNF4alpha and LRH-1 cooperate in regulating Cyp7a1 in vivo. J Biol Chem 2012; 287: 41334–41341.
Bhutta HY, White W, Liu Y, Rajpal N, Way J, Rajpal D et al. Effect of roux-en-y gastric bypass surgery on fecal bile acid excretion in normal and diabetic rats. Diabetes 2013; 62: A520 (abstract 2018P).
Li JV, Ashrafian H, Bueter M, Kinross J, Sands C, le Roux CW et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut 2011; 60: 1214–1223.
Ashrafian H, Li JV, Spagou K, Harling L, Masson P, Darzi A et al. Bariatric surgery modulates circulating and cardiac metabolites. J Proteome Res 2014; 13: 570–580.
Osto E, Doytcheva P, Corteville C, Spliethoff K, Bueter M, Rohrer L et al. Increased plasma Glucagon Like Peptide-1 improves endothelial dysfunction immediately after Roux-en-Y gastric bypass prior to body weight loss inhibiting the c-Jun N-terminal Protein Kinase Signaling. Eur Heart J 2013; 34: 119 (abstract P617).
Werling M, Vincent RP, Cross GF, Marschall HU, Fandriks L, Lonroth H et al. Enhanced fasting and post-prandial plasma bile acid responses after Roux-en-Y gastric bypass surgery. Scand J Gastroenterol 2013; 48: 1257–1264.
Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 2014; 63: 891–902.
Steinert RE, Peterli R, Keller S, Meyer-Gerspach AC, Drewe J, Peters T et al. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity (Silver Spring) 2013; 21: E660–E668.
Gerhard GS, Styer AM, Wood GC, Roesch SL, Petrick AT, Gabrielsen J et al. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care 2013; 36: 1859–1864.
Simonen M, Dali-Youcef N, Kaminska D, Venesmaa S, Kakela P, Paakkonen M et al. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obes Surg 2012; 22: 1473–1480.
Jansen PL, van Werven J, Aarts E, Berends F, Janssen I, Stoker J et al. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis 2011; 29: 48–51.
Dirksen C, Jorgensen NB, Bojsen-Moller KN, Kielgast U, Jacobsen SH, Clausen TR et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int J Obes (Lond) 2013; 37: 1452–1459.
Alam M, Agenor KK, Wang G, Reilly D, Vincent R, Alaghband-Zadeh J et al. Raised plasma bile acids concentrations are related to change of GLP-1 two years after gastric bypass surgery (GBP) in patients with type 2 diabetes. Obesity 2011; 19: S156 (abstract 499P).
Myronovych A, Kirby M, Seeley RJ, Kohli R . Sleeve gastrectomy in obese mice results in elevated serum bile acids and reduced hepatic steatosis that correlate with weight loss post surgery. Gastroenterology 2012; 142: S13 (abstract 50).
Cummings BP, Bettaieb A, Graham JL, Stanhope KL, Kowala M, Haj FG et al. Vertical sleeve gastrectomy improves glucose and lipid metabolism and delays diabetes onset in UCD-T2DM rats. Endocrinology 2012; 153: 3620–3632.
Myronovych A, Kirby M, Ryan KK, Zhang W, Jha P, Setchell KD et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring) 2014; 22: 390–400.
Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014; 509: 183–188.
Myronovych A, Salazar-Gonzalez RM, Miles L, Ryan KK, Seeley RJ, Kohli R . Short Heterodimer Partner (SHP) is necessary for the improvement in NASH after sleeve gastrectomy in obese mice. Hepatology 2013; 58: 234A–236A (abstract 54).
Stefater MA, Sandoval DA, Chambers AP, Wilson-Perez HE, Hofmann SM, Jandacek R et al. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology 2011; 141: 939–949 e1-4.
Kohli R, Myronovych A, Xanthakos S, Ryan KK, Jenkins T, Salazar-Gonzalez RM et al. Sleeve gastrectomy in mice and humans results in weight loss independent suppression of hepatic bile acid synthesis. Gastroenterology 2013; 144: S319–S320 (abstract Sa1849).
Haluzikova D, Lacinova Z, Kavalkova P, Drapalova J, Krizova J, Bartlova M et al. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity (Silver Spring) 2013; 21: 1335–1342.
Belgaumkar AP, Vincent RP, Carswell KA, Dew T, Mitry RR, Le Roux CW et al. Bile acid absorption does not change after laparoscopic sleeve gastrectomy. Obes Surg 2011; 21: 1106 (abstract P199).
Belgaumkar AP, Vincent RP, Carswell KA, Dew T, Mitry RR, Le Roux CW et al. Plasma bile acid profile is not altered by laparoscopic sleeve gastrectomy. Obes Surg 2011; 21: 1072 (abstract P78).
Mencarelli A, Renga B, D'Amore C, Santorelli C, Graziosi L, Bruno A et al. Dissociation of intestinal and hepatic activities of FXR and LXRalpha supports metabolic effects of terminal ileum interposition in rodents. Diabetes 2013; 62: 3384–3393.
Cummings BP, Bettaieb A, Graham JL, Kim J, Ma F, Shibata N et al. Bile-acid-mediated decrease in endoplasmic reticulum stress: a potential contributor to the metabolic benefits of ileal interposition surgery in UCD-T2DM rats. Dis Model Mech 2013; 6: 443–456.
Cummings BP, Strader AD, Stanhope KL, Graham JL, Lee J, Raybould HE et al. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the UCD-T2DM rat. Gastroenterology 2010; 138: 2437–46, 2446 e1.
Habegger KM, Al-Massadi O, Heppner KM, Myronovych A, Holland J, Berger J et al. Duodenal nutrient exclusion improves metabolic syndrome and stimulates villus hyperplasia. Gut 2014; 63: 1238–1246.
Cohen DE . Balancing cholesterol synthesis and absorption in the gastrointestinal tract. J Clin Lipidol 2008; 2: S1–S3.
West KL, Zern TL, Butteiger DN, Keller BT, Fernandez ML . SC-435, an ileal apical sodium co-dependent bile acid transporter (ASBT) inhibitor lowers plasma cholesterol and reduces atherosclerosis in guinea pigs. Atherosclerosis 2003; 171: 201–210.
Li Q, Chen L, Yang Z, Ye Z, Huang Y, He M et al. Metabolic effects of bariatric surgery in type 2 diabetic patients with body mass index<35 kg/m2. Diabetes Obes Metab 2012; 14: 262–270.
Corradini SG, Eramo A, Lubrano C, Spera G, Cornoldi A, Grossi A et al. Comparison of changes in lipid profile after bilio-intestinal bypass and gastric banding in patients with morbid obesity. Obes Surg 2005; 15: 367–377.
Heffron SP, Singh A, Zagzag J, Youn HA, Underberg JA, Fielding GA et al. Laparoscopic gastric banding resolves the metabolic syndrome and improves lipid profile over five years in obese patients with body mass index 30-40 kg/m(2.). Atherosclerosis 2014; 237: 183–190.
Milone M, Lupoli R, Maietta P, Di Minno A, Bianco P, Ambrosino P et al. Lipid profile changes in patients undergoing bariatric surgery: A comparative study between sleeve gastrectomy and mini-gastric bypass. Int J Surg 2015; 14C: 28–32.
Griffo E, Nosso G, Lupoli R, Cotugno M, Saldalamacchia G, Vitolo G et al. Early improvement of postprandial lipemia after bariatric surgery in obese type 2 diabetic patients. Obes Surg 2014; 24: 765–770.
Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev 2000; 14: 2819–2830.
Houten SM, Watanabe M, Auwerx J . Endocrine functions of bile acids. EMBO J 2006; 25: 1419–1425.
Prieur X, Coste H, Rodriguez JC . The human apolipoprotein AV gene is regulated by peroxisome proliferator-activated receptor-alpha and contains a novel farnesoid X-activated receptor response element. J Biol Chem 2003; 278: 25468–25480.
Claudel T, Inoue Y, Barbier O, Duran-Sandoval D, Kosykh V, Fruchart J et al. Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology 2003; 125: 544–555.
Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B . Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol 2003; 17: 259–272.
Lee J, Seok S, Yu P, Kim K, Smith Z, Rivas-Astroza M et al. Genomic analysis of hepatic farnesoid X receptor binding sites reveals altered binding in obesity and direct gene repression by farnesoid X receptor in mice. Hepatology 2012; 56: 108–117.
Heni M, Wagner R, Ketterer C, Bohm A, Linder K, Machicao F et al. Genetic variation in NR1H4 encoding the bile acid receptor FXR determines fasting glucose and free fatty acid levels in humans. J Clin Endocrinol Metab 2013; 98: E1224–E1229.
Kreymann B, Williams G, Ghatei MA, Bloom SR . Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 1987; 2: 1300–1304.
Yabe D, Seino Y . Two incretin hormones GLP-1 and GIP: comparison of their actions in insulin secretion and beta cell preservation. Prog Biophys Mol Biol 2011; 107: 248–256.
Parker HE, Wallis K, le Roux CW, Wong KY, Reimann F, Gribble FM . Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br J Pharmacol 2012; 165: 414–423.
Adrian TE, Gariballa S, Parekh KA, Thomas SA, Saadi H, Al Kaabi J et al. Rectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia 2012; 55: 2343–2347.
Van der Schueren BJ, Homel P, Alam M, Agenor K, Wang G, Reilly D et al. Magnitude and variability of the glucagon-like peptide-1 response in patients with type 2 diabetes up to 2 years following gastric bypass surgery. Diabetes Care 2012; 35: 42–46.
Salehi M, Prigeon RL, D'Alessio DA . Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 2011; 60: 2308–2314.
Alam ML, Van der Schueren BJ, Ahren B, Wang GC, Swerdlow NJ, Arias S et al. Gastric bypass surgery, but not caloric restriction, decreases dipeptidyl peptidase-4 activity in obese patients with type 2 diabetes. Diabetes Obes Metab 2011; 13: 378–381.
Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H . Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism 2009; 58: 1400–1407.
Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 2004; 145: 2594–2603.
Jimenez A, Casamitjana R, Viaplana-Masclans J, Lacy A, Vidal J . GLP-1 action and glucose tolerance in subjects with remission of type 2 diabetes after gastric bypass surgery. Diabetes Care 2013; 36: 2062–2069.
Sato M, Shibata C, Kikuchi D, Ikezawa F, Imoto H, Sasaki I . Effects of biliary and pancreatic juice diversion into the ileum on gastrointestinal motility and gut hormone secretion in conscious dogs. Surgery 2010; 148: 1012–1019.
Ullmer C, Alvarez Sanchez R, Sprecher U, Raab S, Mattei P, Dehmlow H et al. Systemic bile acid sensing by G protein-coupled bile acid receptor 1 (GPBAR1) promotes PYY and GLP-1 release. Br J Pharmacol 2013; 169: 671–684.
Wu T, Bound MJ, Standfield SD, Gedulin B, Jones KL, Horowitz M et al. Effects of rectal administration of taurocholic acid on glucagon-like peptide-1 and peptide YY secretion in healthy humans. Diabetes Obes Metab 2013; 15: 474–477.
le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 2007; 246: 780–785.
Koop I, Schindler M, Bosshammer A, Scheibner J, Stange E, Koop H . Physiological control of cholecystokinin release and pancreatic enzyme secretion by intraduodenal bile acids. Gut 1996; 39: 661–667.
Lee WJ, Chen CY, Chong K, Lee YC, Chen SC, Lee SD . Changes in postprandial gut hormones after metabolic surgery: a comparison of gastric bypass and sleeve gastrectomy. Surg Obes Relat Dis 2011; 7: 683–690.
Keitel V, Gorg B, Bidmon HJ, Zemtsova I, Spomer L, Zilles K et al. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia 2010; 58: 1794–1805.
Ryan KK, Kohli R, Gutierrez-Aguilar R, Gaitonde SG, Woods SC, Seeley RJ . Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology 2013; 154: 9–15.
Morton GJ, Matsen ME, Bracy DP, Meek TH, Nguyen HT, Stefanovski D et al. FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest 2013; 123: 4799–4808.
Bueter M, Lowenstein C, Olbers T, Wang M, Cluny NL, Bloom SR et al. Gastric bypass increases energy expenditure in rats. Gastroenterology 2010; 138: 1845–1853.
Nestoridi E, Kvas S, Kucharczyk J, Stylopoulos N . Resting energy expenditure and energetic cost of feeding are augmented after Roux-en-Y gastric bypass in obese mice. Endocrinology 2012; 153: 2234–2244.
Faria SL, Faria OP, Cardeal Mde A, Ito MK, Buffington C . Diet-induced thermogenesis and respiratory quotient after Roux-en-Y gastric bypass surgery: a prospective study. Surg Obes Relat Dis 2014; 10: 138–143.
Schmidt JBS, Gregersen NTG, Pedersen SP, Nielsen MSN, Nielsen LVN, Holst JJH et al. The effect of Roux-en-Y gastric bypass surgery on energy expenditure and Appetite: A Randomized Human Study including ‘Pair fed’ control subjects. Obesity Facts 2013; 6: 28 (abstract T5:OS1.5).
Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006; 439: 484–489.
Zhang JH, Nolan JD, Kennie SL, Johnston IM, Dew T, Dixon PH et al. Potent stimulation of fibroblast growth factor 19 expression in the human ileum by bile acids. Am J Physiol Gastrointest Liver Physiol 2013; 304: G940–G948.
Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 2002; 143: 1741–1747.
Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006; 313: 1137–1140.
Holmes E, Li JV, Athanasiou T, Ashrafian H, Nicholson JK . Understanding the role of gut microbiome-host metabolic signal disruption in health and disease. Trends Microbiol 2011; 19: 349–359.
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI . An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444: 1027–1031.
Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI . Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 2005; 102: 11070–11075.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI . Microbial ecology: human gut microbes associated with obesity. Nature 2006; 444: 1022–1023.
Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010; 18: 190–195.
Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013; 500: 541–546.
Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E et al. Dietary intervention impact on gut microbial gene richness. Nature 2013; 500: 585–588.
Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011; 141: 1773–1781.
Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol 2013; 58: 949–955.
Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013; 341: 1241214.
Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012; 143: 913–6 e7.
Kong LC, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr 2013; 98: 16–24.
Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 2010; 59: 3049–3057.
Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A 2009; 106: 2365–2370.
Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM . Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 2013; 5: 178ra41.
Larsson E, Tremaroli V, Lee YS, Koren O, Nookaew I, Fricker A et al. Analysis of gut microbial regulation of host gene expression along the length of the gut and regulation of gut microbial ecology through MyD88. Gut 2012; 61: 1124–1131.
MacHado AD, Forster-Fromme K, Mitra S, Friedrich A, Kramer K, Huson DH et al. Competence network obesity: An integrated multi -omics approach to study the guest-host metabolic interaction during restrictive obesity intervention. Obesity Facts 2012; 5: 4–5 (abstract FV 2.5).
Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR . Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci U S A 2008; 105: 13580–13585.
Miyata M, Yamakawa H, Hamatsu M, Kuribayashi H, Takamatsu Y, Yamazoe Y . Enterobacteria modulate intestinal bile acid transport and homeostasis through apical sodium-dependent bile acid transporter (SLC10A2) expression. J Pharmacol Exp Ther 2011; 336: 188–196.
Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci USA 2011; 108: 4523–4530.
Midtvedt T, Norman A, Nygaard K . Bile acid transforming micro-organisms in rats with an intestinal blind segment. Acta Pathol Microbiol Scand 1969; 77: 162–166.
Kuribayashi H, Miyata M, Yamakawa H, Yoshinari K, Yamazoe Y . Enterobacteria-mediated deconjugation of taurocholic acid enhances ileal farnesoid X receptor signaling. Eur J Pharmacol 2012; 697: 132–138.
Ostlund MP, Lu Y, Lagergren J . Risk of obesity-related cancer after obesity surgery in a population-based cohort study. Ann Surg 2010; 252: 972–976.
Li JV, Reshat R, Wu Q, Ashrafian H, Bueter M, le Roux CW et al. Experimental bariatric surgery in rats generates a cytotoxic chemical environment in the gut contents. Front Microbiol 2011; 2: 183.
Santarelli RL, Pierre F, Corpet DE . Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr Cancer 2008; 60: 131–144.
Kanamoto R, Azuma N, Suda H, Saeki T, Tsuchihashi Y, Iwami K . Elimination of Na+-dependent bile acid transporter from small intestine by ileum resection increases [correction of increase] colonic tumorigenesis in the rat fed deoxycholic acid. Cancer Lett 1999; 145: 115–120.
Houghton PW, Owen RJ, Henly PJ, Mortensen NJ, Hill MJ, Williamson RC . Experimental colonic carcinogenesis after gastric surgery. Br J Surg 1990; 77: 774–778.
Adami GF, Papadia FS, Marinari GM, Camerini GB, Scopinaro N . Does biliopancreatic diversion carry increased risk for colorectal cancer? A cohort study. Obes Surg 2008; 18: 212–215.
Mahawar KK, Carr WR, Balupuri S, Small PK . Controversy surrounding 'mini' gastric bypass. Obes Surg 2014; 24: 324–333.
Musella M, Milone M . Still ‘controversies’ about the mini gastric bypass? Obes Surg 2014; 24: 643–644.
Modica S, Murzilli S, Salvatore L, Schmidt DR, Moschetta A . Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res 2008; 68: 9589–9594.
Giordano C, Catalano S, Panza S, Vizza D, Barone I, Bonofiglio D et al. Farnesoid X receptor inhibits tamoxifen-resistant MCF-7 breast cancer cell growth through downregulation of HER2 expression. Oncogene 2011; 30: 4129–4140.
Goldberg AA, Titorenko VI, Beach A, Sanderson JT . Bile acids induce apoptosis selectively in androgen-dependent and -independent prostate cancer cells. PeerJ 2013; 1: e122.
Acknowledgements
The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. JM Kinross acknowledges funding from the Academy of Medical Sciences, clinical lecturer starter grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Penney, N., Kinross, J., Newton, R. et al. The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: a systematic review. Int J Obes 39, 1565–1574 (2015). https://doi.org/10.1038/ijo.2015.115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.115
This article is cited by
-
Multi-omic phenotyping reveals host-microbe responses to bariatric surgery, glycaemic control and obesity
Communications Medicine (2022)
-
Factors associated with resolution of type-2 diabetes mellitus after sleeve gastrectomy in obese adults
Scientific Reports (2021)
-
Changes in Body Composition and Biochemical Parameters Following Laparoscopic One Anastomosis Gastric Bypass: 1-Year Follow-Up
Obesity Surgery (2021)
-
Cholecystectomy - a potential selection bias in studies assessing the metabolic effects of bariatric surgeries
Scientific Reports (2020)
-
Long-term diabetes outcomes after bariatric surgery—managing medication withdrawl
International Journal of Obesity (2019)