Abstract
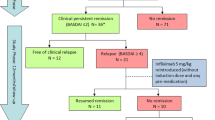
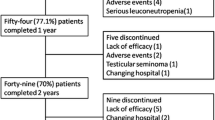
Specific antagonists of tumor necrosis factor (TNFα) have rapidly gain popularity for the treatment of ankylosing spondylitis (AS). The dose of etanercept has not been determined in Asia, especially in Korea. This study was designed to investigate the maintaining effect of low-dose (25 mg/week) etanercept in Korean patients with AS and after discontinuation, the duration to be aggravated. Patients who had active AS [Bath AS Disease Activity Index (BASDAI) ≥4] were treated with 50 mg of etanercept per week for 3 months. After that, for 6 months, the patients were treated with 25 mg of etanercept per week. We evaluated the serum erythrocyte sediment rate (ESR), C-reactive protein (CRP), and BASDAI every 1 month for 3 months and every 2–3 months during the remaining 6 months. After all schedules of treatment were finished, we reevaluated ESR, CRP, and BASDAI every 4 months until recurrence. Twenty-seven AS patients received etanercept. Twenty-three patients completed treatment for 3 months with a dose of 50 mg/week. Among them, 18 completed for 6 months with a dose of 25 mg/week and discontinued. Mean age was 30.0 ± 5.4 years and mean disease duration was 7.5 ± 6.5 years. These 18 patients were evaluated for BASDAI, ESR, and CRP every 4 weeks. After discontinuation, mean duration to recur was 9.2 ± 6.1 weeks. Twenty-five milligrams of etanercept per week is effective enough to maintain remission in AS. After discontinuation, this effect was maintained by using a dose of 50 mg of etanercept per week.

Similar content being viewed by others
References
Malaviya AN, Mehra NK, Adhar G et al (1979) HLA B27 in patients with seronegative spondarthritides. J Rheumatol 6:413–416
Mitsui H, Sonozaki H (1981) Ankylosing spondylitis in Japan. Ryumachi 21(Suppl):45–49
Murata H, Inoue T (1993) Ankylosing spondylitis. Nippon Rinsho 51(Suppl):938–945
Hukuda S, Minami M, Saito T et al (2001) Spondyloarthropathies in Japan: nationwide questionnaire survey performed by the Japan Ankylosing Spondylitis Society. J Rheumatol 28:554–559
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27:361–368
Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A (1994) A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 21:2286–2291
Schwartzman S, Morgan GJ Jr (2004) Does route of administration affect the outcome of TNF antagonist therapy? Arthritis Res Ther 6(Suppl 2):S19–S23
Genovese MC, Bathon JM, Fleischmann RM et al (2005) Longterm safety, efficacy, and radiographic outcome with etanercept treatment in patients with early rheumatoid arthritis. J Rheumatol 32:1232–1242
Moreland LW, Cohen SB, Baumgartner SW et al (2001) Long-term safety and efficacy of etanercept in patients with rheumatoid arthritis. J Rheumatol 28:1238–1244
Johnsen AK, Schiff MH, Mease PJ et al (2006) Comparison of 2 doses of etanercept (50 vs 100 mg) in active rheumatoid arthritis: a randomized double blind study. J Rheumatol 33:659–664
Jois RN, Leeder J, Gibb A et al (2006) Low-dose infliximab treatment for ankylosing spondylitis-clinically- and cost-effective. Rheumatology (Oxford) 45(12):1566–1569
Brandt J, Khariouzov A, Listing J et al (2003) Six-month results of a double-blind, placebo-controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis Rheum 48:1667–1675
Acknowledgements
I specially thank my patients who were enrolled in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, SH., Lee, YA., Hong, SJ. et al. Etanercept 25 mg/week is effective enough to maintain remission for ankylosing spondylitis among Korean patients. Clin Rheumatol 27, 179–181 (2008). https://doi.org/10.1007/s10067-007-0674-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-007-0674-2